In January 2025, the University of Chicago and Deerfield Management announced the launch of Hyde Park Discovery, a collaboration aiming to advance the development of new medicines and other life-saving treatments for disease.

The University of Chicago and Deerfield Management Launch Hyde Park Discovery to Accelerate Translation of Research Into Life-Saving Treatments
Deerfield Management commits up to $130 million to support UChicago drug discovery and development
CHICAGO and NEW YORK, Jan. 28, 2025 /PRNewswire/ — The University of Chicago and healthcare investment firm Deerfield Management announced today the launch of Hyde Park Discovery, a collaboration aiming to advance the development of new medicines and other life-saving treatments for disease.
Over the next ten years, Deerfield will provide up to $130 million in targeted funding, as well as operational and scientific expertise to advance UChicago discoveries with the potential to become novel therapies. The strategic agreement brings together UChicago’s leading research and innovation capabilities across several key therapeutic areas and Deerfield’s experience in healthcare product development.
“The Biological Sciences Division at UChicago and the UChicago Medicine health system comprise a distinct environment that brings together basic researchers studying fundamental biology and physicians who provide advanced patient care. It is crucial to have the kind of support and innovative capabilities that Deerfield brings to accelerate discoveries and create meaningful results for our community and the world,” said Mark Anderson, dean of the Biological Sciences Division and the Pritzker School of Medicine and executive vice president for Medical Affairs at UChicago.
A joint steering committee made up of leadership from UChicago, the Polsky Center for Entrepreneurship and Innovation, and Deerfield’s scientific team will evaluate projects based on several criteria with the goal of achieving Investigational New Drug readiness on an accelerated timeline. This operating model has the potential to bring new drugs to the market more quickly and cost-effectively.
“Our partnership with the University of Chicago and the Polsky Center underscores Deerfield’s conviction in the significant translational potential of the research that takes place there,” said James Flynn, managing partner at Deerfield. “In joining forces, we look forward to advancing compelling therapeutics together.”
“Bringing innovative ideas and technologies from the University of Chicago ecosystem to the world is the mission of the Polsky Center, and collaborations such as this help us make this goal a reality,” said Samir Mayekar, associate vice president and managing director of the Polsky Center for Entrepreneurship and Innovation, which houses the University’s official technology transfer office. “Backed by Deerfield’s scientific capabilities, we are optimistic that this agreement and the work that follows will result in new potential treatments and cures getting into the hands of the patients who need them.”
The University of Chicago is on the forefront of groundbreaking research and clinical development with a leading academic medical system, more than 160 interdisciplinary institutes and centers, and 50 state-of-the-art core facilities. World-class faculty bring together the fundamentals of medicine and advanced technology with expertise in computer science, AI, materials science, chemistry, and more.
“We are thrilled about partnering with Deerfield in the pursuit of commercializing discoveries made at the University of Chicago that have the potential to impact patient care,” said Scott Oakes, professor and vice dean of clinical science research in the Biological Sciences Division at UChicago. “This investment attests to the incredible science being done here and helps to advance our mission of bringing the fruits of that research to the world.”
About the University of Chicago
The University of Chicago is a leading academic and research institution that has driven new ways of thinking since its founding in 1890. As an intellectual destination, the University draws scholars and students from around the world to its campuses and centers around the globe. The University provides a distinctive educational experience and research environment, empowering individuals to challenge conventional thinking and pursue field-defining research that produces new understanding and breakthroughs with global impact.
About the Polsky Center for Entrepreneurship and Innovation
The Polsky Center for Entrepreneurship and Innovation advances innovative ideas and technologies from the University of Chicago ecosystem to the world. We are dedicated to supporting students, faculty, staff, and alumni, as well as other entrepreneurs and community-based small business owners in navigating the complex process of bringing new products, services, and research to market. For more information, visit polsky.uchicago.edu.
About Deerfield Management
Deerfield is an investment management firm committed to advancing healthcare through investment, information, and philanthropy. The Firm works across the healthcare ecosystem to connect people, capital, ideas, and technology in bold, collaborative, and inclusive ways. For more information, please visit www.deerfield.com.
Media Contact
Jessica Sagers, Head of Communications, Deerfield Management
[email protected], 332-323-0100
SOURCE Deerfield Management Company, L.P.

VeritaScience
In January 2024, Washington University in St. Louis and Deerfield Management announced the launch of VeritaScience, a private R&D collaboration designed to advance the discovery, clinical development and commercialization of promising therapeutic and diagnostic candidates with potential to benefit human health.

Washington University and Deerfield Management Launch VeritaScience to Drive Drug Discovery
Deerfield Management makes 10-year commitment of up to $130 million to accelerate translation of Washington University discoveries to improve human health
New York, New York, – January 16, 2024 Washington University in St. Louis and Deerfield Management (“Deerfield”), a healthcare investment firm, today announced the launch of VeritaScience, a new private R&D collaboration designed to advance the discovery, clinical development and commercialization of promising therapeutic and diagnostic candidates with potential to benefit human health.
To support projects that originate from the collaboration, Deerfield has committed up to $130 million over the next 10 years, along with functional expertise, through a newly formed company. Washington University’s investigators will have the opportunity to work with Deerfield’s internal team, which has expertise across the drug development continuum from discovery through commercialization. VeritaScience is named to honor Washington University’s motto “Per Veritatem Vis,” which means “Strength Through Truth” in Latin.
The VeritaScience collaboration complements the university’s existing efforts in drug development and its innovative culture. Project proposals can be designed around any disease indication. Washington University’s investigators will submit project proposals to VeritaScience and its scientific review team, which is led by Deerfield experts and guided by the university’s Office of Technology Management and Washington University School of Medicine’s (“WashU Medicine”) business development team. Accepted drug discovery projects will receive a development plan aimed at achieving Investigational New Drug readiness and may be eligible for additional funding and support for the creation of separate, start-up companies.
Washington University is known worldwide for its innovative research programs in the medical sciences, aimed at improving human health. The university currently is ranked #7 in National Institutes of Health (NIH) funding among U.S. universities, with $633 million received in the 2023 federal fiscal year. This includes nearly $584 million in funding for research initiatives at WashU Medicine, which currently is ranked #2 in NIH funding among U.S. medical schools.
“The exciting collaboration with Deerfield Management to create VeritaScience represents yet another major approach to leveraging the research capabilities of Washington University,” said David H. Perlmutter, MD, the George and Carol Bauer Endowed Dean of Washington University School of Medicine, executive vice chancellor for medical affairs, and the Spencer T. and Ann W. Olin Distinguished Professor. “Deerfield has extensive experience in academic-industry partnerships, and this agreement allows us to further advance the ‘virtuous cycle of academic medicine’ and our commitment to translating ever more of the landscape-altering discoveries happening in our labs into tools and therapies that will concretely improve human health and alleviate suffering.”
The VeritaScience collaboration is an example of Deerfield’s engagement with research institutions. Currently, Deerfield’s network includes nearly 30 leading research institutions and medical centers, which aims to bring the healthcare ecosystem together to collaborate and learn from each other. Together with its research partners, Deerfield has provided funding and expertise to create spin-off companies that support key avenues from concept to spin-out of novel therapeutic discoveries.
“Throughout the process of forming VeritaScience, what has stood out to our team at Deerfield is the forward-thinking and innovation-focused Washington University culture,” said James Flynn, managing partner of Deerfield. “We intend to equip the researchers of selected projects with flexible capital, expertise, and operational support to help them realize their discoveries’ potential.”
ABOUT WASHINGTON UNIVERSITY
Washington University in St. Louis is counted among the world’s leaders in teaching, research, patient care and service to society. The university draws students and faculty to St. Louis from more than 100 countries and all 50 states, the District of Columbia, Guam, Puerto Rico and the Virgin Islands. The total student body is approximately 14,000 undergraduate, graduate and professional students. Washington University has been affiliated with 25 Nobel laureates, many of whom did a significant portion of their award-winning work at the university. The university offers more than 90 programs and almost 1,500 courses leading to bachelor’s, master’s and doctoral degrees in a broad spectrum of traditional and interdisciplinary fields, with additional opportunities for minor concentrations and individualized programs.
WashU Medicine is a global leader in academic medicine, including biomedical research, patient care and educational programs with 2,800 faculty. Its National Institutes of Health (NIH) research funding portfolio is the second largest among U.S. medical schools, has grown more than 40% in the last six years and, together with institutional investment, WashU Medicine commits well over $1 billion annually to basic and clinical research innovation and training. Its faculty practice is consistently within the top five in the country, with more than 1,800 faculty physicians practicing at over 65 locations and who are also the medical staffs of Barnes-Jewish and St. Louis Children’s hospitals of BJC HealthCare.
ABOUT DEERFIELD MANAGEMENT
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. The Firm works across the healthcare ecosystem to connect people, capital, ideas and technology in bold, collaborative and inclusive ways. For more information, please visit www.deerfield.com.
Media Contact:
Anthony Karamourtopoulos
Diane Duke Williams

Funding Models
Sample from 2008 to April 2023:


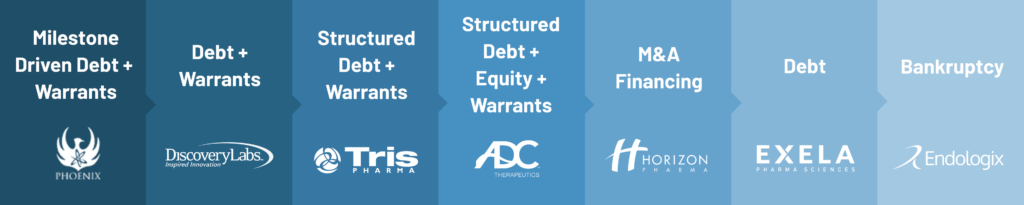
Please note that the listed transactions are a sample of the types of deals that can be created as well as just a sampling of the companies we have worked with over the time period from 2008 to 2023.

Melinta Therapeutics Successfully Completes Financial Restructuring
Company is well-positioned for success as it emerges from chapter 11
NEW YORK, NY, April 20, 2020—Commercial-stage antibiotics company Melinta Therapeutics (“Melinta”) and healthcare investment firm Deerfield Management Company, L.P. (“Deerfield”), announced today that Melinta has successfully completed its financial restructuring and has emerged from Chapter 11.
In accordance with the pre-negotiated Chapter 11 plan of reorganization, Melinta is now privately owned by affiliates of Deerfield and has eliminated its debt obligations, resulting in a well-financed and strongly positioned anti-infectives company with plans for future growth.
Melinta will continue to actively supply, distribute, and support its four marketed products for the treatment of certain antibiotic-resistant infections: Vabomere® (meropenem and vaborbactam), Orbactiv® (oritavancin), Minocin® (minocycline) for Injection and Baxdela® (delafloxacin). In addition, with its new, solid financial footing, Melinta expects to enhance its portfolio with the addition of new commercial and clinical-stage pipeline candidates in support of its mission of serving the critical needs of patients in the hospital and hospital ecosystem.
“We welcome this partnership with Deerfield in continuing to best serve the needs of patients in the hospital and look forward to the new opportunities for innovation and growth that this partnership will bring,” said Jennifer Sanfilippo, Interim Chief Executive Officer and Director of the reorganized company. “Our antibiotics will remain a central component of Melinta’s portfolio, including our core brands Vabomere® and Orbactiv®, and we are excited at the prospect of augmenting this important portfolio with products that address high-need therapeutic areas.”
In partnership with the Melinta team, Deerfield intends to leverage its operational, business development, data analytics and market research expertise in order to continue to accelerate the growth and expansion of Melinta’s product portfolio.
“The Melinta team has demonstrated an ability to successfully deliver important antibiotics to treat serious infections and has shown resilience and dedication during the most challenging of times,” said Deerfield Partner Jonathan Leff. “Covid-19 is a wake-up call regarding the dangers of infectious diseases and the need for innovative anti-infective products to serve the public health. We are delighted to join Melinta in this journey.”
-####-
Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
Melinta Therapeutics, Inc. is dedicated to saving lives threatened by the global public health crisis of drug resistant bacterial infections through the development and commercialization of novel antibiotics that provide new therapeutic solutions. Its four marketed products are Vabomere® (meropenem and vaborbactam), Orbactiv® (oritavancin), Minocin® (minocycline) for Injection and Baxdela® (delafloxacin). This portfolio provides Melinta with the unique ability to provide providers and patients with a range of solutions that can meet the tremendous need for novel antibiotics treating serious infections. For additional information, including product information, visit www.melinta.com.
Contact:
Deerfield Management
Karen Heidelberger, 212-692-7140, [email protected]
Melinta Therapeutics
Susan Blum, 312-767-0296, [email protected]

Large-Scale Cell and Gene Therapy Contract Development and Manufacturing Organization to Launch in PA
The Center for Breakthrough Medicines expected to relieve the industry’s production constraints, providing patients better access to treatments
(King of Prussia, PA, and New York, NY, January 22, 2020)—The Discovery Labs and Deerfield Management Company have formed The Center for Breakthrough Medicines, a Contract Development and Manufacturing Organization (CDMO) and specialty investment company, to alleviate the critical lack of capacity that is preventing patients from accessing critically needed cell and gene therapies. The CDMO is occupying over 40 percent of The Discovery Labs’ 1.6 million square foot biotech, healthcare and life sciences campus in King of Prussia, PA.
The CDMO provides preclinical through commercial manufacturing of cell and gene therapies and component raw materials. It offers process development, plasmid DNA, viral vectors, cell banking, cell processing, and support testing capabilities all under one roof. The immense $1.1 billion facility will provide instant capacity as the largest known single source for accelerating the delivery and affordability of lifesaving and life-changing therapies from the bench to the patient’s bedside.
The Company has initiated a substantial hiring effort targeting the best and brightest of the life sciences community including, experts in CGMP manufacturing. The Company expects to hire over 2,000 team members within the next 30 months.
The CDMO has retained Nucleus Careers, a cloud-based specialty life sciences human capital recruiting and retention management expert, to buildout the entire team. Nucleus has proprietary recruiting and retention software designed for large scale human capital buildouts of high growth companies.
In addition to developing the world’s largest single-point cell and gene therapy manufacturing facility, The Discovery Labs is establishing THE COLONY which will provide custom built discovery labs, breakthrough funding, sponsored research agreements, housing and relocation for the world’s leading iconic experts in cell and gene therapy.
THE COLONY will seek to work hand in hand with scientists from both academic and pharmaceutical institutions to unlock and expedite groundbreaking therapies.
Marco A. Chacón, Ph.D., Founder of Paragon Bioservices and Chairman of The Discovery Labs states, “musicians, artists, members of religious communities and great thinkers throughout time have formed colonies where freedom of thought and expression combined with unlimited dreams and potential have resulted in the world’s greatest accomplishments. The United States of America is a perfect example.” Dr. Chacón went on to say, “the goal of THE COLONY is to unshackle the potential of the world’s greatest scientific minds.”
The ability for the industry’s greatest scientists to cohabitate, collaborate, cooperate, and communicate via technology and in person will create an exponential therapeutic “X FACTOR.” THE COLONY seeks to unlock institutional barriers prohibiting the world’s greatest scientists from moving at a pace necessary in today’s ever-changing therapeutic revolution. THE COLONY will partner with the institutions where the scientists currently work by providing equity, license fees, and revenue sharing.
“The Center for Breakthrough Medicines will be serving companies from the earliest stages through commercialization. Its exceptional scale and offering will quickly relieve the production bottleneck for advanced therapies by reducing the time, complexity, and cost of commercializing vitally needed gene and cell therapies,” noted Audrey Greenberg, Board Member and Executive Managing Director for The Discovery Labs.
The addition of this end-to-end manufacturing capability is expected to significantly enhance the offerings of The Discovery Labs in an area that has become one of the largest life sciences hubs in the world. Renovations are underway to construct a total of 86 plasmid, viral vector production, universal cell processing, CGMP testing, process development and cell banking suites. The viral vector and cell processing suites will be fully compliant with both U.S. Food and Drug Administration and European Medicines Agency standards. All suites will offer the flexibility to meet client-specific workflows and will be able to adapt quickly to meet demand. The Company is in the process of reserving capacity now for late 2020.
“Today brilliant scientists are advancing an unprecedented number of gene and cell therapy drug candidates. The real tragedy, however, is a scarcity of manufacturing know-how, which is complex and expensive,” said Alex Karnal, Partner and Managing Director of Deerfield Management and a Board Member of the Discovery Labs. “With its visionary business model, it is hoped that The Center for Breakthrough Medicines will help realize the promise of cell and gene therapies in time to treat the many patients who need them.”
The Discovery Labs provides a central campus where the world’s greatest scientists can collaborate on new therapeutic discoveries to eradicate diseases affecting small and large segments of the global population. The Center for Breakthrough Medicines will work with these leaders, life sciences companies, large pharmaceutical companies, and academic and government institutions.
This new manufacturing capability is a transformational addition to The Discovery Labs market offering and dovetails with The Discovery Labs biotech incubator, Unite IQ. Unite IQ offers immediate space to emerging life sciences companies and scientists giving them the ability to grow from startup to enterprise company on one campus. The incubator and accelerator space at Unite IQ provides a comprehensive home for startups with every resource needed to initiate business operations. Unite IQ tenants are expected to utilize the discovery, development, testing, and manufacturing capabilities of the Center for Breakthrough Medicines with seamless forward integration of processes and analytics, and seamless tech transfer from research lab to large scale production
The Emerging Field of Cell and Gene Therapy in Pennsylvania
The demand for clinical and commercial manufacturing capacity is acute and expected to remain that way. The current shortfall in manufacturing for cell and gene therapies is severely underserved with few approved products. There are currently approximately 1,100 advanced therapies in the pipeline pending FDA approval. This will greatly increase highly skilled manufacturing demand. Dr. Peter Marks, Director of the FDA Center for Biologics Evaluation and Research, states, “what keeps me up at night is will we be able to manufacture these on a scale that will allow us to bring the benefit of these therapies to patients?” He further added that “if we can help see cost of goods and ability to manufacture reproducibly improve, I think that’ll be a big thing.” All of this adds up to a supply constrained market that The Center for Breakthrough Medicines aims to help address.
With the potential to treat and even cure disabling, and deadly diseases, gene and cell therapies are ushering in a new era of medicine. These therapies may eventually be able to cure genetic conditions, such as cystic fibrosis, hemophilia A, and a range of cancers. The Philadelphia area has become the epicenter for the flourishing field of gene and cell therapy. Research from CBRE currently ranks the market among the top biotech clusters for medical research and health services. The cluster has become known worldwide as “Cellicon Valley” for its leadership in research and development of this rapidly evolving field. The Discovery Lab’s suburban Philadelphia location offers a talent rich environment due to the area’s preponderance of large pharmaceutical companies and the Philadelphia region’s position boasting the top 10 universities and primary school systems in nation.
Over the past three years, multiple Philadelphia companies have received approvals for major breakthroughs in cell and gene therapy. In 2017, the U.S. FDA approved the first-ever CAR-T cell therapy, Novartis’s Kymriah, which originated at the University of Pennsylvania. Shortly thereafter, the FDA gave landmark approval for the first-ever gene therapy to treat a genetic blindness condition to Spark Therapeutics, a start-up founded by researchers at Children’s Hospital of Philadelphia. These discoveries and others in the pipeline are attracting billions of dollars of venture capital. The Greater Philadelphia Region set a recent record in venture capital financing.
The Discovery Labs Center for Breakthrough Medicines joins more than 25 healthcare, life sciences and tech-enabled companies that already call The Discovery Labs King of Prussia home.
Contact Audrey Greenberg at [email protected] for more information about development services, manufacturing capacity, incubator space or leasing information at the property.
About The Discovery Labs
Part of MLP Ventures, The Discovery Labs is a global provider of world-class cGMP manufacturing, turnkey laboratory solutions, critical materials and office space that support therapeutic products and services to the biotechnology and pharmaceutical industry so that groundbreaking medicines get to the patients that need them. The location in eastern King of Prussia is a prototype for a global rollout of The Discovery Labs, providing Big Pharma, emerging life sciences, consumer and technology companies flexible, end-to-end technical real estate and business infrastructure for the customer’s entire lifecycle from discovery to delivery, including manufacturing capacity. It is the first fully integrated environment that merges technology and life sciences under one roof to drive innovation.
About Deerfield Management
Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
Media Contact:
Tony DeFazio, DeFazio Communications
(o) 484-534-3306 (c) 484-410-1354
[email protected]
Karen Heidelberger, Deerfield Management Company
212-551-1600
[email protected]

Science to the Street (formerly Women in Science)
Launched in 2019 by Deerfield, the Women in Science Translational Research Symposia were created to clarify the process and route to commercialization for women scientists and in turn, help prepare them to advance their discoveries to market. The program is committed to promoting diversity of perspectives in innovation and changing this paradigm through knowledge and network creation.
In 2022, the Women in Science Translational Research Symposia formally embedded within Advancium Health Network, an independent non-profit organization launched by Deerfield Management and the Deerfield Foundation and continues to be supported by all three organizations.