Sample from 2008 to April 2023:


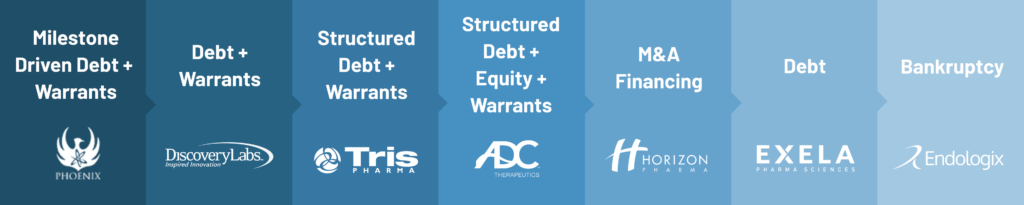
Please note that the listed transactions are a sample of the types of deals that can be created as well as just a sampling of the companies we have worked with over the time period from 2008 to 2023.

Sample from 2008 to April 2023:



Please note that the listed transactions are a sample of the types of deals that can be created as well as just a sampling of the companies we have worked with over the time period from 2008 to 2023.
“We have spent the past 20 years on a journey to take the roots and foundation of Deerfield and evolve into an integrated, diversified organization which we believe is fundamentally advancing healthcare through supporting both for-profit as well as not-for-profit work.”
Download the article to read more
“Every drug since 2010, was invented by a university and by partnering with over 20 large universities, we are teaming up with the source of innovation and hopefully that leads to a differentiated return,” said Deerfield’s Jim Flynn
Download the article to read more
This article should not be construed as an offer to sell, or a solicitation to buy, any security or instrument in, or to participate in any investment strategy with any funds, vehicles or accounts sponsored or managed by Deerfield Management Company, L.P. (“Deerfield”) or any portfolio company in which Deerfield has invested. The list of portfolio companies provided herein is not a complete list of all current or historical portfolio companies. To learn more, please see the Portfolio Company section of Deerfield’s website at www.Deerfield.com. PE Hub has been compensated for the right to redistribute this article. PE Hub is not affiliated with Deerfield and was not compensated to publish this article on Deerfield’s behalf. While Deerfield believes the content of the article is accurate, Deerfield makes no representation or warranty as to the accuracy or completeness of the information provided herein. It should not be assumed that any current, former, or future investments were or will be profitable. There is no guarantee that other opportunities will have similar characteristics or results to those described in this article.

February 15, 2022 01:56 PM Eastern Standard Time
NEW YORK–(BUSINESS WIRE)–Deerfield Management and the Deerfield Foundation today announced the formation of Advancium Health Network, an independent charitable organization. Its mission is to advance human equity through innovation and education by bringing together diverse thought leaders, entrepreneurs, and mission-focused problem solvers to create cures and solutions that would not be furthered through a commercial lens.
“There are critical innovations that have the potential to create a compelling impact to patients in need if supported through thought leadership and funding.”
To fulfill its mission, Advancium embraces a two-pronged approach:
“Advancium Health Network can and will make a significant difference in addressing some of the most pressing healthcare challenges today that might not otherwise be solved,” said James Flynn, Managing Partner, Deerfield Management and Cure Founder. “There are critical innovations that have the potential to create a compelling impact to patients in need if supported through thought leadership and funding.”
Advancium identifies and develops potential revolutionary medical discoveries that address rare diseases, important medical device technologies, disease in children, among many other healthcare needs. The organization will build initiatives to address the gender and racial gaps that exist in the healthcare and financial service industries with diversity programs created by Deerfield Management, including the Deerfield Fellows Program, Break into the Boardroom, and Women in Science.
“We need to source all of the best thinking and do so in a way that reflects the diversity of the scientific ecosystem if we’re to make a meaningful impact on global human health,” said Ron Mitchell, Advancium board member and CEO of Humanity Health. “I’m excited to see the impact we can have.”
Ron Mitchell will be joined on Advancium’s Board by Jeffrey Kaplan, Founder and Chairman of the A to Z Impact Foundation, David Doneson, Chief Executive Officer of the American Committee for the Weizmann Institute of Science, Olivier Elemento, Ph.D., the Director of the Englander Precision Medicine Institute at Weill Cornell Medicine, and Mark Veich, the Executive Director of the Deerfield Foundation, who will also serve as the President of Advancium.
About Deerfield Management
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. The Firm works across the healthcare ecosystem to connect people, capital, ideas and technology in bold, collaborative and inclusive ways.
For more information, please visit www.deerfield.com.
About Deerfield Foundation
An affiliate of Deerfield Management, the Deerfield Foundation is a New York City-based not-for-profit organization whose mission is to strive to improve health, accelerate innovation and promote human equity. Since its inception in 2007, the Foundation has formed numerous partnerships and invested in the advancement of children’s health, from clinics in the South Bronx to Nepal. Funds are provided through employee contributions and directly from Deerfield’s profits. https://deerfield.com/deerfield-foundation
About Advancium Health Network
Advancium Health Network is a volunteer-driven 501(c)(3) charitable organization mission-focused on advancing human equity and accelerating innovation to improve health for people globally.
Advancium Health recognizes that a highly linked, more diverse community of healthcare innovators and providers is foundational to tackling health disparities, expanding treatment options, and finding cures, especially for rare diseases. The network is built to bring together people from academic medical centers, research universities, healthcare companies, start-ups, financial institutions, government, and nonprofit organizations. https://AdvanciumHealth.org
Eden Zaslow
212-300-1800
[email protected]

November 03, 2021 10:17 AM Eastern Daylight Time
NEW YORK–(BUSINESS WIRE)–Deerfield Management Company, a New York City-based healthcare investment firm today announced its second annual XSeed Award, a program designed to support New York City early-stage life science, minority-led startups. This year’s XSeed Award will be granted to individuals working on neurodegeneration research development projects.
“Funding translational product development by underrepresented communities is critical to help spark innovation,” said James E. Flynn, Managing Partner at Deerfield. “Broader diversity allows for new and different vantage points to be incorporated into the innovation and startup community, and the varied perspectives indisputably create better ideas and solutions. NYC is one of the most diverse communities in the world, and so must be our early-stage life science community.”
According to Pitchbook, life science ventures led by women and minorities still face a substantial funding gap. Female founders in the U.S. raised only 2.2 percent of total venture capital funding in 2020 and funding to Black and Latinx founders equate to only 2.4% of capital raised from 2015 through August 2020.
The underrepresented scientists and entrepreneurs working on important healthcare projects in NYC are actively furthering discoveries but too often lack support to progress more cures for patients. Through fostering this community of individuals, NYC can help change the disease narrative of the Biotech Industry.
“The New York City Economic Development Corporation is overseeing the City’s $1 billion investment in life sciences, and for us it’s about breaking down barriers and creating equal opportunity in the field. We applaud Deerfield Management for its efforts to support minority-led life science research and early-stage ventures through the second XSeed Award program,” said Rachel Loeb, President and CEO at NYCEDC. “Now, underrepresented scientists and entrepreneurs can apply for much needed funding to help yield the discoveries and cures of tomorrow.”
Each year, the award is launched with a specific focus, selecting five teams that will be awarded a total of up to $500,000. Finalists present their work to a panel which includes: Claire Pomeroy, MD, President and CEO of the Albert and Mary Lasker Foundation; Nancy Thornberry, Founding CEO at Kallyope, Inc.; Elise Wang, Partner at Deerfield; Joseph Pearlberg, Vice President of Scientific Affairs at Deerfield, H.C. Huang, Senior Director, Deerfield Discovery and Development at Deerfield, and Jennifer Laird, VP, Search & Evaluation – Pain & Neurodegeneration at Eli Lilly.
The deadline for submission is January 7, 2022, and the winners will be announced in March. To learn more about the XSeed Award’s applicant eligibility criteria, visit www.xseedaward.com.
“Being recognized as a winner of the XSeed Award means more than just advancing my research and work, it has enriched my learnings and expanded my networking opportunities.” said Chandrabali Ghose-Paul, Founder and CEO of Bioharmony Therapeutics, a winner of the inaugural XSeed Awards in 2021. “Interacting with Deerfield and a group of my fellow recipients strengthens our fight against infectious diseases through knowledge and support.”
“The XSeed Award is what true executive sponsorship looks like,” remarked Ron Mitchell, CEO of Humanity Health, a career acceleration and talent sourcing platform for underrepresented healthcare leaders and entrepreneurs which is supported by Deerfield. “Sponsorship is about amplifying, connecting, endorsing and supporting leaders in their work. All four of those elements are deeply ingrained in this amazing opportunity provided by Deerfield.”
Deerfield created the XSeed Award in tandem with its development of Cure, the life science campus it built in partnership with New York City Economic Development Corporation (NYCEDC). The Cure campus at 345 Park Avenue South in Midtown Manhattan is bringing together innovators from academia, government, industry, and the not-for-profit sectors to advance human health and accelerate the fight against disease. Residents have access to move-in-ready laboratory space, engineering and computing space, as well as other amenities and support services.
ABOUT DEERFIELD
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. The Firm works across the healthcare ecosystem to connect people, capital, ideas and technology in bold, collaborative and inclusive ways. For more information, please visit www.deerfield.com.
Contacts
Media Contact:
Caroline Drucker
[email protected]

(New York, NY, April 20, 2021)—Deerfield Management Company, a New York City-based healthcare investment firm, today announced the grant recipients of its XSeed Award, a new program designed to support early-stage life science startups. Each of the five New York City-based award winners, a majority of which were women or other underrepresented founders, will receive a $100,000 grant to further projects focused on infectious diseases.
The 2021 recipients of Deerfield’s inaugural XSeed Award are:
“This year has been unlike any other in so many ways, not the least of which is the unprecedented advancement of medical science,” said Joseph Pearlberg, MD, Ph.D., Vice President of Scientific Affairs at Deerfield. “Our inaugural group of XSeed Awardees will support some of New York City’s most talented researchers and scientists with a focus on developing cures for infectious diseases. As we begin to rebound from the pandemic, we cannot become complacent – support for new ideas and vaccines should remain a global priority.”
The XSeed Award was created by Deerfield as a result of the partnership announced in September 2019 between NYC Economic Development Corporation (NYCEDC) and Deerfield to develop the Cure life science campus. The 12-story vertical campus is bringing together innovators from academia, government, industry, and the not-for-profit sectors under one roof to advance human health and accelerate the fight against disease. Occupants will benefit from state-of-the-art laboratories, engineering and computing space, as well as amenities and support services.
“New York City has all the critical pieces to become a world-class life sciences hub, including diversity, and it’s imperative that we strategically support underrepresented minority groups to ensure equity throughout our efforts to grow the sector,” said NYCEDC Acting President Rachel Loeb. “Together with Deerfield, we are supporting early-stage entrepreneurs across all sectors and from all backgrounds. This critical financial support complements the services provided within the Cure campus and is a natural extension of our partnership.”
The Cure campus is part of LifeSci NYC, a $500 million commitment to establish New York City as a global leader in the life sciences. The opening of the Cure includes the relocation of Deerfield’s corporate headquarters to the site at 345 Park Avenue South in New York City (from its past location of 780 Third Avenue). With this endeavor, Deerfield has invested to create the transformative life sciences campus. In addition, Deerfield announced its programmatic efforts at the Cure, starting with a virtual lecture series led by experts covering topics, including COVID-19 Vaccine Confidence: Engaging with the Community for Improved Uptake and “Are you OK?” Having Conversations about Mental Health at Work.
“Congratulations to the recipients of the inaugural XSeed Awards! The Life Sciences is a growing industry in New York City, one that can help grow our economy and employment base as we recover from the pandemic. More importantly, we need to continue to ensure that these 21st Century job and entrepreneurship opportunities are inclusive, providing access to innovators from underrepresented communities. I look forward to seeing Deerfield’s initiatives and LifeSci NYC programs catapult New York City to the forefront of innovation and diversity in biotech,” said Councilwoman Carlina Rivera.
“The grants awarded to New York City-based life science startups will bring immense return on our investment. This is a tremendous step forward for developing the life science vertical campus as New York City becomes a global leader in advancing human health and fighting against diseases”, said Council Member Paul A. Vallone.
Bridging the Critical Funding Gap
With a 20+ year commitment, the XSeed Award program will help address a critical funding gap, bridging promising translational research to marketplace and commercial success.
Each year, the award will have a specific focus with Deerfield selecting five teams that will be awarded a total of up to $500,000. Finalists presented their startups to a panel made up by: Claire Pomeroy, MD, President and CEO of the Albert and Mary Lasker Foundation; Nancy Thornberry, CEO at Kallyope, Inc.; James E. Flynn, Managing Partner of Deerfield; Elise Wang, Partner at Deerfield; and Joseph Pearlberg, MD, Ph.D., Vice President of Scientific Affairs at Deerfield, among others.
Importantly, in addition to receiving award funding, the winning teams will join a two-year cohort of their fellow awardees. The teams will be provided with peer-learning and office hours with leading investors, entrepreneurs, and business experts. Through the XSeed Award cohort, it is anticipated that these startups will bolster their network and significantly raise their visibility. Graduates of each cohort are expected to in turn serve as mentors and coaches for future cohorts, further strengthening the entrepreneurial community in New York City.
Deerfield’s Commitment to Promoting Equality
The life sciences sector is comprised of many mission-driven companies in fields like biotechnology, pharmaceuticals, biomedical technologies, medical devices, and biochemistry that work to translate scientific research into cures and treatments that save and improve lives.
However, funding for women and other minority-led life science research and ventures face a significant gap. Female founders raised only 2.3 percent of total venture capital funding in 2020. Black applicants are 10 percent less likely than whites to be awarded NIH Funding, and women of color, more broadly, have raised less than 1 percent of all venture capital funding since 2009.
With its rich history of developing and leading programs that support diversity, additional educational programming will continue, including Deerfield’s growing LifeSci NYC Fellows and Break into the Boardroom programs. In addition, in 2019 Deerfield introduced a new effort, Women in Science, focused on training women on how to commercialize their novel discoveries and create companies.
Deerfield announced in September 2019 that it intended to commit more than $2 billion in research and seed funding by 2030 to develop much-needed new and innovative medicines and treatment solutions. Deerfield expects this world-class infrastructure and funding will contribute to the prevention, cure or management of dozens of still deadly and debilitating diseases.
To learn more about the XSeed Award, visit www.xseedaward.com.
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. For more information, please visit www.deerfield.com
An affiliate of Deerfield Management Company based in the heart of New York City, the Cure is a 12-story vertical innovations campus boasting laboratories, lecture, and office space, as well as technology and other amenities for physician-scientists/entrepreneurs across the life science industry, including academic institutions and other nonprofits, to accelerate their novel work. For more information, please visit https://cure.345pas.com/
Media Contact:
BerlinRosen
Erin Holin
917-232-0701
(NEW YORK CITY, NY, April 6, 2021) – Deerfield Management Company (Deerfield), a healthcare investment management firm focused on advancing healthcare through investment, information and philanthropy, today released “Gender Disparity Among Venture-backed Healthcare Companies and Their Investor Base,” a paper that analyzes the gender composition of healthcare companies’ corporate boards, as well as that of the investment teams finding, diligencing and mentoring these companies.
In an analysis of 140 healthcare companies that had raised substantial venture financing and published the identity of their board members on their corporate website, Deerfield found that:
● 48.5% of companies had no female board members.
● Women make up about 10% of board director roles in venture-backed healthcare companies.
● Among the most active investment firms in the healthcare space, only about 20% of investment professionals are female.
● Of the six organizations analyzed that have 50% or more female board membership, all but one are helmed by female CEOs.
The paper’s authors, Christine Livoti, Director at the Deerfield Institute at Deerfield and Leslie Henshaw, Partner on the Healthcare Services team at Deerfield, set out to understand the gender composition of venture-backed healthcare companies that operate in the industry subsectors in which Deerfield invests.
Most of the research on diversity in leadership focuses on public companies that are required to report their diversity data. As such, information on private healthcare companies’ board composition is largely unavailable to the public. Livoti and Henshaw established a proprietary database of healthcare companies in the therapeutics, medical devices and diagnostics, healthcare information technology and healthcare services subsectors to identify the gender composition of their corporate boards.
The paper also examines the gender composition of the top 50 healthcare investors, based on the number of healthcare transactions in which they participated. Overall, women make up only about 20% of the investment professionals in this group of firms, and even this modest percentage likely overestimates the proportion of senior female investment professionals relative to their junior counterparts.
“Deerfield sits at the intersection of healthcare and investing, two spheres in which women are vastly underrepresented,” said Leslie Henshaw, Partner at Deerfield and co-author of the paper. “The findings of our report are unsurprising, but still deeply disappointing. With this research, our goal is to help our peers understand the considerable gender imbalance on their own teams is having a direct impact on the board composition of the companies they fund, increasing the imperative to seek out diverse independent board members. We are committed to elevating female and marginalized leaders across the healthcare ecosystem, increasing equity and accessibility throughout an industry in which the role of women – as caregivers, decision makers and patients – is so pronounced.”
Given the outsized role that investors play in board seat allocation and placement, the gender diversity of investment firms cannot be ignored in the context of gender diversity of private company boards.
“While gender composition of public companies, or even a broader swath of private companies agnostic of sector has been examined before, uniquely we examined the gender composition of private healthcare companies’ leadership and their investors,” said Christine Livoti, Director at The Deerfield Institute and co-author of the study. “We hope these findings will serve as a wakeup call for private companies in our industry to examine the gender composition of their leadership and work harder to diversify and grow more equitably across all levels of their organizations.”
The paper’s findings aim to hold companies and investors accountable, encourage greater female representation and advance initiatives for investors to diversify the portfolio companies in which they invest.
“Gender Disparity Among Venture-backed Healthcare Companies and Their Investor Base” is one of Deerfield’s several initiatives to improve representation in the healthcare ecosystem. Deerfield oversees Break into the Boardroom, a program that was launched in 2016 to help promote greater representation of female healthcare executives on boards within the public, private, and non-profit sectors. Break into the Boardroom provides senior female healthcare executives with training and guidance intended to help them obtain their first board role.
In the coming months, a programmatic effort will take place at the Cure, a 12-story vertical innovations campus in New York City, to dive deeper on this topic.
“We see significant value in the medtech industry and are excited to extend our investment in medtech with this newly formed partnership. Coridea’s track record makes its team an ideal partner to innovate, evaluate, and foster new ideas from concept to commercial enterprise,” stated Steve Hochberg, Partner at Deerfield. “As a New York-based firm, we are proud to partner with the team from Coridea as it brings innovative ideas and enterprise-building capabilities into the Cure ecosystem. This will serve to further establish the Northeast region and New York City as a center for healthcare innovation.”
Formed by proven industry leaders, the incubator will provide its companies with discipline and expertise in a collaborative ecosystem. Grounded by years of industry experience, the team will be able to efficiently move projects through key early-stage milestones including needs assessment, concept generation, rapid prototyping, and pre-clinical and clinical evaluation. Deerfield Catalyst will source new projects and companies through internal ideation and external collaboration with academic or individual partners.
“For sustainable commercial success, innovation must address a clear clinical need, whether it be streamlining a procedure to reduce cost or developing a novel intervention to treat more patients. At Deerfield Catalyst we plan to use agile best practices to efficiently advance promising clinical ideas and companies through the development lifecycle,” commented Howard Levin, M.D., CEO of Coridea and Chief Executive of Deerfield Catalyst. “We see a bright future ahead and are excited to become a critical part of the Cure healthcare ecosystem.”
The partnership aims to launch 10 new companies over the next five years. Structured to support company formation and growth, the incubator includes funding for operating expenses, early idea generation, and project and company evaluation. The medtech incubator hub and newly formed companies will be housed in the Cure building at 345 Park Avenue South in Manhattan.
ABOUT BREAK INTO THE BOARDROOM
Break into the Boardroom aims to promote greater representation of female healthcare executives on boards within the public, private, and non-profit sectors. Break into the Boardroom was founded by Deerfield and Oxeon based on a shared belief that their organizations are uniquely positioned not only to cultivate new female board candidates but also to connect these women with concrete governance opportunities. Deerfield and Oxeon focus on identifying talent, cultivating companies, and deploying capital within the healthcare ecosystem. For more information, please visit breakintotheboardroom.org.
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. For more information, please visit www.deerfield.com
CONTACT
BerlinRosen
(New York, NY, December 22, 2020)—Deerfield Management Company, a New York City-based healthcare investment firm, today announced the launch of the X-Seed Award, a new program designed to support early-stage life science startups. Award winners will be women and other minority founders in New York City.
The X-Seed Award was created by Deerfield as a result of the partnership announced in September 2019 between NYCEDC and Deerfield to develop the Cure life science campus, which is slated to open in January 2021. The 12-story vertical campus is bringing together innovators from academia, government, industry, and the not-for-profit sectors under one roof to advance human health and accelerate the fight against disease. Occupants will benefit from state-of-the-art laboratories, engineering and computing space, as well as other amenities and support services.
“As the City looks towards the future, we are committed to a recovery that puts equity at the center,” said James Patchett, President and CEO of New York City Economic Development Corporation. “The Deerfield X-seed Award is a major milestone towards achieving that goal. By supporting early stage entrepreneurs of all backgrounds, we can advance the City’s position as a leader in life sciences innovation for years to come.”
The X-Seed Award program, with a 20+ year commitment, will provide funding for a diverse population of the most talented researchers and entrepreneurs in New York City. It will help address a critical funding gap, bridging promising translational research to marketplace and commercial success.
Each year, the award will have a specific focus with Deerfield selecting five teams that will be awarded a total of up to $500,000. Finalists will have an opportunity to present their startups to a panel which includes: Claire Pomeroy, MD, President and CEO of the Albert and Mary Lasker Foundation; Nancy Thornberry, CEO at Kallyope, Inc.; James E. Flynn, Managing Partner of Deerfield; Elise Wang, Principal at Deerfield; and Joseph Pearlberg, Vice President of Scientific Affairs at Deerfield, among others. For 2021, given the state of the pandemic, the focus will be on infectious disease.
Deadline for submission is January 29,, 2021 and the winners will be announced in March. To learn more about the X-Seed Fund’s applicant eligibility criteria, visit www.xseedaward.com
In addition to receiving award funding, the winning teams will join a two-year cohort of their fellow awardees. The teams will be provided with peer-learning, and office hours with leading investors, entrepreneurs, and business experts. Through the X-Seed Award cohort, it is anticipated that these startups will bolster their network and significantly raise their visibility. Graduates of each cohort will in turn serve as mentors and coaches for future cohorts, further strengthening the entrepreneurial community in New York City.
The life sciences sector is comprised of mission-driven companies in fields like biotechnology, pharmaceuticals, biomedical technologies, medical devices, and biochemistry that work to translate scientific research into cures and treatments that save and improve lives.
However, funding for women and other minority-led life science research and ventures face a significant gap. Female founders raised only 2.3 percent of total venture capital funding in 2018. Black applicants are 10 percent less likely than whites to be awarded NIH Funding and women of color, more broadly, have raised less than 1 percent of all venture capital funding since 2009.
The X-Seed Award was designed to disrupt this trend to create a stronger, more equitable New York. “If we are to continue to make progress in the fight against disease, we must make supporting and mentoring New York’s most talented and diverse scientists a priority, laying the groundwork for future generations to come,” said Karen Heidelberger, Partner and Chief Partnership and Communications Officer at Deerfield. “I can think of no better way to do that than to create the X-Seed Award program. We look forward to this exciting journey, as New York becomes a leading life science capital.”
With its rich history of developing and leading programs that support diversity, additional educational programming will continue, including Deerfield’s growing LifeSci NYC Fellows and Break into the Boardroom programs. In addition, in 2019 Deerfield introduced a new effort, Women in Science, focused on training women on how to commercialize their novel discoveries and create companies.
The campus is part of LifeSci NYC, a $500 million commitment to establish New York City as a global leader in the life sciences. The opening of the Cure will include the relocation of Deerfield’s corporate headquarters to the site at 345 Park Avenue South in New York City (from its current location of 780 Third Avenue). With this endeavor, Deerfield has invested $635 million to create the transformative life sciences campus.
Deerfield announced in September 2019 that it intended to commit more than $2 billion in research and seed funding by 2030 to develop much-needed new and innovative medicines and treatment solutions. Deerfield expects this world-class infrastructure and funding will contribute to the prevention, cure or management of dozens of still deadly and debilitating diseases.
Deerfield is an investment management firm committed to advancing healthcare through investment, information and philanthropy. For more information, please visit www.deerfield.com
An affiliate of Deerfield Management Company that’s based in the heart of New York City, the Cure is a 12-story vertical innovations campus boasting laboratories, lecture, and office space, as well as technology and other amenities for physician-scientists/entrepreneurs across the life science industry, including academic institutions and other nonprofits, to accelerate their novel work. For more information, please visit https://cure.345pas.com/
CONTACT
Karen Heidelberger, 212-551-1600, [email protected]

An affiliate of Deerfield Management, the Deerfield Foundation is a New York City-based not-for-profit organization whose mission is to strive to improve health, accelerate innovation and promote human equity. Since its inception in 2007, the Foundation has formed numerous partnerships and invested in the advancement of children’s health, from clinics in the South Bronx to Nepal. Funds are provided through employee contributions and directly from Deerfield’s profits.
Mark Veich, Executive Director
Kerri Seacord, Treasurer
Julianna Santamaria, Executive Assistant
Abdul Khan, Program Committee Member
Adam Grossman, Program Committee Member
Ankita Shah, Program Committee Member
Bhumika Patel, Program Committee Member
Cecilia Perez, Program Committee Member
Chris Freeland, Program Committee Member
Danielle Rosato, Program Committee Member
David Griffith, Program Committee Member
Elise Wang, Program Committee Member
Emma Giegerich, Program Committee Member
Gabby Briganti, Program Committee Member
George Lytle, Program Committee Member
Giselle Pineda, Program Committee Member
H.C. Huang, Program Committee Member
Huijun Wang, Program Committee Member
Ivo Lorenz, Program Committee Member
John Limanto, Program Committee Member
Kate Lazar, Program Committee Member
Lisa Nordfors, Program Committee Member
Lizzy Baer, Program Committee Member
Luci Albertson, Program Committee Member
Mark Shtilerman, Program Committee Member
Mike Hurley, Program Committee Member
Moses Adubi, Program Committee Member
Nehan Chatoor, Program Committee Member
Nelson Barriocanal, Program Committee Member
Nicole Delgado, Program Committee Member
Rachel Chapin, Program Committee Member
Saiyara Fahmi, Program Committee Member
Sam Becker, Program Committee Member
Terence Fox-Karnal, Program Committee Member
Tori Fleek, Program Committee Member

At CURE. — Deerfield’s innovation campus on 345 Park Avenue South — we’re transforming healthcare. Here, innovators from across the industry and around the world work shoulder to shoulder to develop treatments to eliminate deadly diseases and create new healthcare delivery models to lower cost and improve care for those in need.
For CURE. inquiries, please email us