Sample from 2008 to April 2023:


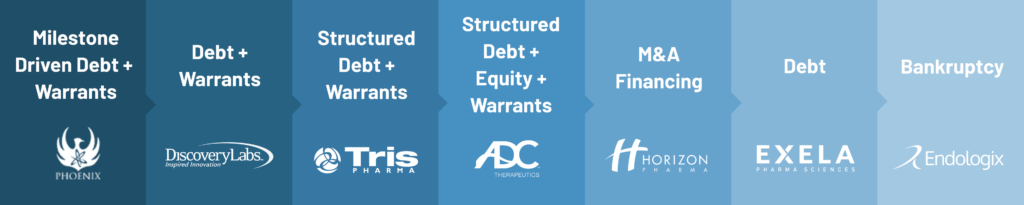
Please note that the listed transactions are a sample of the types of deals that can be created as well as just a sampling of the companies we have worked with over the time period from 2008 to 2023.

Sample from 2008 to April 2023:



Please note that the listed transactions are a sample of the types of deals that can be created as well as just a sampling of the companies we have worked with over the time period from 2008 to 2023.

NEW YORK, NY, April 20, 2020—Commercial-stage antibiotics company Melinta Therapeutics (“Melinta”) and healthcare investment firm Deerfield Management Company, L.P. (“Deerfield”), announced today that Melinta has successfully completed its financial restructuring and has emerged from Chapter 11.
In accordance with the pre-negotiated Chapter 11 plan of reorganization, Melinta is now privately owned by affiliates of Deerfield and has eliminated its debt obligations, resulting in a well-financed and strongly positioned anti-infectives company with plans for future growth.
Melinta will continue to actively supply, distribute, and support its four marketed products for the treatment of certain antibiotic-resistant infections: Vabomere® (meropenem and vaborbactam), Orbactiv® (oritavancin), Minocin® (minocycline) for Injection and Baxdela® (delafloxacin). In addition, with its new, solid financial footing, Melinta expects to enhance its portfolio with the addition of new commercial and clinical-stage pipeline candidates in support of its mission of serving the critical needs of patients in the hospital and hospital ecosystem.
“We welcome this partnership with Deerfield in continuing to best serve the needs of patients in the hospital and look forward to the new opportunities for innovation and growth that this partnership will bring,” said Jennifer Sanfilippo, Interim Chief Executive Officer and Director of the reorganized company. “Our antibiotics will remain a central component of Melinta’s portfolio, including our core brands Vabomere® and Orbactiv®, and we are excited at the prospect of augmenting this important portfolio with products that address high-need therapeutic areas.”
In partnership with the Melinta team, Deerfield intends to leverage its operational, business development, data analytics and market research expertise in order to continue to accelerate the growth and expansion of Melinta’s product portfolio.
“The Melinta team has demonstrated an ability to successfully deliver important antibiotics to treat serious infections and has shown resilience and dedication during the most challenging of times,” said Deerfield Partner Jonathan Leff. “Covid-19 is a wake-up call regarding the dangers of infectious diseases and the need for innovative anti-infective products to serve the public health. We are delighted to join Melinta in this journey.”
-####-
Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
Melinta Therapeutics, Inc. is dedicated to saving lives threatened by the global public health crisis of drug resistant bacterial infections through the development and commercialization of novel antibiotics that provide new therapeutic solutions. Its four marketed products are Vabomere® (meropenem and vaborbactam), Orbactiv® (oritavancin), Minocin® (minocycline) for Injection and Baxdela® (delafloxacin). This portfolio provides Melinta with the unique ability to provide providers and patients with a range of solutions that can meet the tremendous need for novel antibiotics treating serious infections. For additional information, including product information, visit www.melinta.com.
Contact:
Deerfield Management
Karen Heidelberger, 212-692-7140, [email protected]
Melinta Therapeutics
Susan Blum, 312-767-0296, [email protected]

(King of Prussia, PA, and New York, NY, January 22, 2020)—The Discovery Labs and Deerfield Management Company have formed The Center for Breakthrough Medicines, a Contract Development and Manufacturing Organization (CDMO) and specialty investment company, to alleviate the critical lack of capacity that is preventing patients from accessing critically needed cell and gene therapies. The CDMO is occupying over 40 percent of The Discovery Labs’ 1.6 million square foot biotech, healthcare and life sciences campus in King of Prussia, PA.
The CDMO provides preclinical through commercial manufacturing of cell and gene therapies and component raw materials. It offers process development, plasmid DNA, viral vectors, cell banking, cell processing, and support testing capabilities all under one roof. The immense $1.1 billion facility will provide instant capacity as the largest known single source for accelerating the delivery and affordability of lifesaving and life-changing therapies from the bench to the patient’s bedside.
The Company has initiated a substantial hiring effort targeting the best and brightest of the life sciences community including, experts in CGMP manufacturing. The Company expects to hire over 2,000 team members within the next 30 months.
The CDMO has retained Nucleus Careers, a cloud-based specialty life sciences human capital recruiting and retention management expert, to buildout the entire team. Nucleus has proprietary recruiting and retention software designed for large scale human capital buildouts of high growth companies.
In addition to developing the world’s largest single-point cell and gene therapy manufacturing facility, The Discovery Labs is establishing THE COLONY which will provide custom built discovery labs, breakthrough funding, sponsored research agreements, housing and relocation for the world’s leading iconic experts in cell and gene therapy.
THE COLONY will seek to work hand in hand with scientists from both academic and pharmaceutical institutions to unlock and expedite groundbreaking therapies.
Marco A. Chacón, Ph.D., Founder of Paragon Bioservices and Chairman of The Discovery Labs states, “musicians, artists, members of religious communities and great thinkers throughout time have formed colonies where freedom of thought and expression combined with unlimited dreams and potential have resulted in the world’s greatest accomplishments. The United States of America is a perfect example.” Dr. Chacón went on to say, “the goal of THE COLONY is to unshackle the potential of the world’s greatest scientific minds.”
The ability for the industry’s greatest scientists to cohabitate, collaborate, cooperate, and communicate via technology and in person will create an exponential therapeutic “X FACTOR.” THE COLONY seeks to unlock institutional barriers prohibiting the world’s greatest scientists from moving at a pace necessary in today’s ever-changing therapeutic revolution. THE COLONY will partner with the institutions where the scientists currently work by providing equity, license fees, and revenue sharing.
“The Center for Breakthrough Medicines will be serving companies from the earliest stages through commercialization. Its exceptional scale and offering will quickly relieve the production bottleneck for advanced therapies by reducing the time, complexity, and cost of commercializing vitally needed gene and cell therapies,” noted Audrey Greenberg, Board Member and Executive Managing Director for The Discovery Labs.
The addition of this end-to-end manufacturing capability is expected to significantly enhance the offerings of The Discovery Labs in an area that has become one of the largest life sciences hubs in the world. Renovations are underway to construct a total of 86 plasmid, viral vector production, universal cell processing, CGMP testing, process development and cell banking suites. The viral vector and cell processing suites will be fully compliant with both U.S. Food and Drug Administration and European Medicines Agency standards. All suites will offer the flexibility to meet client-specific workflows and will be able to adapt quickly to meet demand. The Company is in the process of reserving capacity now for late 2020.
“Today brilliant scientists are advancing an unprecedented number of gene and cell therapy drug candidates. The real tragedy, however, is a scarcity of manufacturing know-how, which is complex and expensive,” said Alex Karnal, Partner and Managing Director of Deerfield Management and a Board Member of the Discovery Labs. “With its visionary business model, it is hoped that The Center for Breakthrough Medicines will help realize the promise of cell and gene therapies in time to treat the many patients who need them.”
The Discovery Labs provides a central campus where the world’s greatest scientists can collaborate on new therapeutic discoveries to eradicate diseases affecting small and large segments of the global population. The Center for Breakthrough Medicines will work with these leaders, life sciences companies, large pharmaceutical companies, and academic and government institutions.
This new manufacturing capability is a transformational addition to The Discovery Labs market offering and dovetails with The Discovery Labs biotech incubator, Unite IQ. Unite IQ offers immediate space to emerging life sciences companies and scientists giving them the ability to grow from startup to enterprise company on one campus. The incubator and accelerator space at Unite IQ provides a comprehensive home for startups with every resource needed to initiate business operations. Unite IQ tenants are expected to utilize the discovery, development, testing, and manufacturing capabilities of the Center for Breakthrough Medicines with seamless forward integration of processes and analytics, and seamless tech transfer from research lab to large scale production
The demand for clinical and commercial manufacturing capacity is acute and expected to remain that way. The current shortfall in manufacturing for cell and gene therapies is severely underserved with few approved products. There are currently approximately 1,100 advanced therapies in the pipeline pending FDA approval. This will greatly increase highly skilled manufacturing demand. Dr. Peter Marks, Director of the FDA Center for Biologics Evaluation and Research, states, “what keeps me up at night is will we be able to manufacture these on a scale that will allow us to bring the benefit of these therapies to patients?” He further added that “if we can help see cost of goods and ability to manufacture reproducibly improve, I think that’ll be a big thing.” All of this adds up to a supply constrained market that The Center for Breakthrough Medicines aims to help address.
With the potential to treat and even cure disabling, and deadly diseases, gene and cell therapies are ushering in a new era of medicine. These therapies may eventually be able to cure genetic conditions, such as cystic fibrosis, hemophilia A, and a range of cancers. The Philadelphia area has become the epicenter for the flourishing field of gene and cell therapy. Research from CBRE currently ranks the market among the top biotech clusters for medical research and health services. The cluster has become known worldwide as “Cellicon Valley” for its leadership in research and development of this rapidly evolving field. The Discovery Lab’s suburban Philadelphia location offers a talent rich environment due to the area’s preponderance of large pharmaceutical companies and the Philadelphia region’s position boasting the top 10 universities and primary school systems in nation.
Over the past three years, multiple Philadelphia companies have received approvals for major breakthroughs in cell and gene therapy. In 2017, the U.S. FDA approved the first-ever CAR-T cell therapy, Novartis’s Kymriah, which originated at the University of Pennsylvania. Shortly thereafter, the FDA gave landmark approval for the first-ever gene therapy to treat a genetic blindness condition to Spark Therapeutics, a start-up founded by researchers at Children’s Hospital of Philadelphia. These discoveries and others in the pipeline are attracting billions of dollars of venture capital. The Greater Philadelphia Region set a recent record in venture capital financing.
The Discovery Labs Center for Breakthrough Medicines joins more than 25 healthcare, life sciences and tech-enabled companies that already call The Discovery Labs King of Prussia home.
Contact Audrey Greenberg at [email protected] for more information about development services, manufacturing capacity, incubator space or leasing information at the property.
Part of MLP Ventures, The Discovery Labs is a global provider of world-class cGMP manufacturing, turnkey laboratory solutions, critical materials and office space that support therapeutic products and services to the biotechnology and pharmaceutical industry so that groundbreaking medicines get to the patients that need them. The location in eastern King of Prussia is a prototype for a global rollout of The Discovery Labs, providing Big Pharma, emerging life sciences, consumer and technology companies flexible, end-to-end technical real estate and business infrastructure for the customer’s entire lifecycle from discovery to delivery, including manufacturing capacity. It is the first fully integrated environment that merges technology and life sciences under one roof to drive innovation.
Deerfield is a healthcare investment management firm committed to advancing healthcare through investment, information and philanthropy.
Media Contact:
Tony DeFazio, DeFazio Communications
(o) 484-534-3306 (c) 484-410-1354
[email protected]
Karen Heidelberger, Deerfield Management Company
212-551-1600
[email protected]

Established in 2006, the Deerfield Institute delivers sophisticated and timely market research, which enhances the insights available to the investment team and its partners. The Institute develops and analyzes data to advance understanding of innovations and emerging products, and it also informs on trends within the healthcare market. It has published important scientific findings in peer-reviewed journals to extend learnings to the scientific community and benefit public health at large—and ultimately patients in need. The Institute is guided by Deerfield’s core values: integrity, the pursuit of objective and unbiased research, commitment to excellence, precision and accuracy.

An affiliate of Deerfield Management, the Deerfield Foundation is a New York City-based not-for-profit organization whose mission is to strive to improve health, accelerate innovation and promote human equity. Since its inception in 2007, the Foundation has formed numerous partnerships and invested in the advancement of children’s health, from clinics in the South Bronx to Nepal. Funds are provided through employee contributions and directly from Deerfield’s profits.
Mark Veich, Executive Director
Kerri Seacord, Treasurer
Julianna Santamaria, Executive Assistant
Abdul Khan, Program Committee Member
Adam Grossman, Program Committee Member
Ankita Shah, Program Committee Member
Bhumika Patel, Program Committee Member
Cecilia Perez, Program Committee Member
Chris Freeland, Program Committee Member
Danielle Rosato, Program Committee Member
David Griffith, Program Committee Member
Elise Wang, Program Committee Member
Emma Giegerich, Program Committee Member
Gabby Briganti, Program Committee Member
George Lytle, Program Committee Member
Giselle Pineda, Program Committee Member
H.C. Huang, Program Committee Member
Huijun Wang, Program Committee Member
Ivo Lorenz, Program Committee Member
John Limanto, Program Committee Member
Kate Lazar, Program Committee Member
Lisa Nordfors, Program Committee Member
Lizzy Baer, Program Committee Member
Luci Albertson, Program Committee Member
Mark Shtilerman, Program Committee Member
Mike Hurley, Program Committee Member
Moses Adubi, Program Committee Member
Nehan Chatoor, Program Committee Member
Nelson Barriocanal, Program Committee Member
Nicole Delgado, Program Committee Member
Rachel Chapin, Program Committee Member
Saiyara Fahmi, Program Committee Member
Sam Becker, Program Committee Member
Terence Fox-Karnal, Program Committee Member
Tori Fleek, Program Committee Member

Despite the high aptitude of this representative sample of women leaders, the stark reality is boards are only made up of 20% women. Deerfield and Oxeon founded the Break into the Boardroom program (BiB) in 2015 based on a shared belief that their organizations are uniquely positioned to not only cultivate new women board candidates but to also connect these women with concrete governance opportunities.
Deerfield and Oxeon focus on identifying talent, building companies, and deploying capital within the healthcare ecosystem, which enables BiB to create the perfect intersection of supply and demand. This framework naturally mitigates the classic chicken and egg problem that has proven such an intractable obstacle to the goal of increasing women board representation.
In 2022, BiB welcomed Welsh Carson Anderson & Stowe to its sponsorship team and formally embedded BiB within Advancium Health Network, an independent non-profit organization launched by Deerfield Management and the Deerfield Foundation. Together this newly expanded team aims to broaden the program’s reach by increasing the variety and cadence of its programming and engaging with an ever-widening network of board decision makers.
Please visit the BiB website where you may submit inquiries regarding potential board roles or nominate a female executive from your organization to participate in the program.


At CURE. — Deerfield’s innovation campus on 345 Park Avenue South — we’re transforming healthcare. Here, innovators from across the industry and around the world work shoulder to shoulder to develop treatments to eliminate deadly diseases and create new healthcare delivery models to lower cost and improve care for those in need.
For CURE. inquiries, please email us

New York, NY – January 7, 2019 – Stelexis Therapeutics, LLC announced that it closed a $43 million Series A financing to expand its proprietary platform to discover and selectively target pre‐cancerous stem cells. Deerfield established Stelexis in 2017 together with scientific founders, Ulrich Steidl, Evripidis Gavathiotis, Amit Verma, and Roman Perez‐Soler of Albert Einstein College of Medicine, Montefiore Health, New York and Derrick Rossi of Boston Children’s Hospital, Harvard Medical School. Patrick Doyle serves as the founding CEO, and Keren Paz is the CSO of Stelexis.
Stelexis’ proprietary drug discovery platform identifies the earliest definable pre‐cancerous stem and progenitor cells that lead to the formation of human primary and recurrent tumors for therapeutic intervention and relapse prevention. Stelexis’ mission is to develop novel cancer drugs that selectively target these critical pre‐cancerous events related to both hematopoietic and solid malignancies.
“The ability to identify, isolate, study and screen rare pre‐cancerous stem cells, from within bulk tumors, is an enormous breakthrough that has the potential to change how cancer patients are treated” stated Dr. Steidl. “Our thesis is that targeting cancer at its very origin should not only be effective as first line therapy, but should also lead to long‐lasting remission for patients,” said Dr. Rossi, who, prior to co‐founding Stelexis, has also co‐founded numerous other successful biotechnology companies.
Utilizing Deerfield seed funding and operational support since 2017, Stelexis has established its labs in Albert Einstein College of Medicine facilities, hired key management and is poised to deliver clinical trial data that validate its platform using the proceeds of this Series A round.
“We are thrilled to announce the formation and funding of Stelexis, which has the platform technology to explore the role pre‐cancer conditions play in cancer development and recurrence. The team has an outstanding track record and we look forward to a stream of transformative cancer medicines,” stated Dr. Robert Jackson, director at Stelexis and partner at Deerfield Management.
“Deerfield’s holistic approach to forming, funding and providing operational support to Stelexis has been instrumental in creating a leadership position in a novel targeted approach to treating cancer,” said Patrick Doyle, CEO of Stelexis. “With these funds we are now positioned to execute on our potential to transform patients’ lives.”
Stelexis is a New York‐based cancer therapeutics company, utilizing its proprietary platform to selectively target pre‐cancerous stem cells to discover and develop transformative therapies.
For more information, please visit www.stelexis.com
Deerfield is an investment management firm committed to improving healthcare through investment, information and philanthropy.
For more information, please visit www.deerfield.com
Deerfield Management Company
Karen Heidelberger
212‐551‐1600
[email protected]
Stelexis Therapeutics
Patrick Doyle
[email protected]

Pharma sales representatives (reps) are one of the most integral components to a manufacturer’s commercialization plan. They have long been the voice in determining and shaping the adoption curve of a new drug. However, the “good ol days” are in the rearview mirror for reps as there have been efforts to curb dollars and gifts that reps can shower upon physicians and their staffs, as well as both fewer and lower quality touch points with the decision makers with purchasing power and/or authority on drug choice, particularly when there are multiple options in the same therapeutic category. Here, we will try to put the role of the rep in context and provide some perspective, conceptually, on where the sales model may migrate in the future, and what issues will need to be addressed.
Companies spend significant amounts of time, energy and resources dedicated to planning and building out the sales force optimization plan. Specifically, the commercial team oversees multiple teams to plan the success of the sales rep. Activities that contribute to this success include:
In the past, the sales rep had many tools at their disposal to engage and sell their products to physicians:
The rep had the ability to access the majority of not just physicians, but importantly those with autonomy and decision-making authority, and the nature of the detail (industry lingo for reps providing doctors with the details of a drug, including approved scientific information, benefits, adverse events, etc) evolved around the clinical efficacy and utility of the product. Reps were trained on the mechanism of action of their product as well as competitors, all the key clinical data in the therapeutic area, and how to convey their product’s differentiation. This last part is significant as training also focused on breaking down clinical studies, with successful reps well-trained in how to handle any objections raised by doctors around use of their product (ideally identified ahead of time by the manufacturer’s market research), and able to rattle off why one product may have a better p-value (measure of statistical significance used in clinical trials) or better efficacy at symptom resolution, among other product features.

Reps historically had an environment that was for the most part without many restrictions, which is increasingly no longer the case. The ability of the sales rep to develop a relationship with doctors they called upon was easy and simple due to the tools at their disposal. However, over the past two decades, there have been considerable changes in both the ability to access and the types of activities the reps can use to engage with doctors. These changes have come about in an effort to clamp down on what has been viewed as inappropriate influence over docs by industry reps.
Any payments made to physicians by drug and device companies must be reported under the Physician Payment Sunshine Act, signed into law in 2010 along with the Affordable Care Act, and the data is made public by the Centers for Medicare and Medicaid. While these payments can be as seemingly benign as research grants, it can also include things like travel and accommodations around medical conferences and scientific or advisory board meetings, food and beverage, and consulting fees. Details on the types of payments are included in the public data set. Now the public can see if physicians are receiving what might be perceived as healthy sums of money, which whether innocently or not, can be perceived as doctors being “in the pocket” of big pharma, and a potential PR concern for their institutions.
Most academic medical centers do not allow reps to call on their physicians on site, though there may still be some level of doctor-rep interaction if the doctor maintains an off-campus practice location. One industry report from ZS Associates found that 56% of physicians in the US have either restricted or severely restricted who can visit them, with some specialties even more restrictive. Integrated delivery networks (IDNs), systems where the provider network is also the payer, and group practices, are also increasingly locking out reps. Further, physicians’ prescribing autonomy in IDNs and group practices is not the same as in an independent practice, with decisions around drug purchase and utilization instead made by higher level management.
This trend is likely to continue as cost pressures have made it more and more difficult for independent physician practices to remain financially viable. Independent physician practices have been on the decline since 2000, according to a 2016 analysis by inVentiv Health Consulting. While 57% of physicians were independent in 2000, this decreased to 37% in 2013 and was expected to continue sliding to 33% by the end of 2016.
When reps are still able to get interactions with doctors, the nature of the sales call has shifted from a clinical sell to putting the rep more in the role of a reimbursement navigator. Price never came up as a major topic of the sales detail, whereas now it may be the primary focus of the conversation, as it is often easier for a doctor to simply pick the drug that is cheapest for their patient – “is it covered by insurance and how much will the patient need to pay?” The use of co-pay cards, which reps can drop at the physician office to reduce financial burden for patients (discussed in a past issue of this newsletter), was largely unheard of until more recent years. Still, co-pay cards are not a panacea, and doctors remain skeptical of the overall utility of these cards. Patients often encounter issues at the pharmacy when they try to use these cards, and may ultimately abandon any attempts to get the drug they were prescribed in the first place. These co-pay cards are off-limits to Medicare patients by law (but fair game for those with private insurance), and so might only benefit a fraction of the doctor’s patients.
Put this all together, and reps now have less time to discuss more complicated topics like reimbursement during their detail. The question now to ponder is how will the rep model evolve with the environmental dynamics at play. Specialty reps in the device sector that are required to train docs in the surgical area will continue their business as usual, but there will be a need to evolve the model overall in many sectors.
There is probably not a one size fits all strategy, and that current strategies in the therapeutics sector need to be adjusted to address some of the key challenges outlined below.

Where are we in the continuum with the shift to a more holistic approach? Our observation is that manufacturers acknowledge and discuss that we are at a tipping point, with some having piloted various more integrated approaches to drive behavioral change. Some of these pilot approaches have yielded positive returns but we have not yet seen a full migration over to a new model. What we do believe, however, is that the environmental factors at play will eventually force a significant change for all pharma.
At first, finding any two pieces that might fit together in a complex jigsaw puzzle is a Herculean task. Painstakingly, colors and shapes can be sorted one-by-one into groups that seem to be related. One satisfying match is then followed by another, sometimes preceded by long frustrated periods of searching yet in other moments an almost magical placement and orientation allows for instant recognition. With each piece connected, the picture becomes clearer and the pool of remaining pieces smaller. Each becomes easier to find than the last, and easier to place until at last the puzzle is complete.
So it is with understanding the biologic underpinnings of disease. At first there is a confusing mixture of molecules, interacting with each other in undecipherable pathways. Slowly the different types of molecules are identified, separated and tested to see what they interact with and how. As the function and interactions of each molecule are placed in turn, the picture of causation becomes clearer, and the work to identify the remaining molecular players becomes easier and easier.
Since the unveiling of the human genome nearly 20 years ago, progress in elucidating the underpinnings of disease has occurred at an exponential pace. Gone are the days when insights are shared in only annual gatherings or through publications which can be separated from the work to produce them by years. Instead, this is an age where insights are often shared real time over the internet. In this way, laboratories learn which pieces have been placed in near to real time and the chase to create the finalized picture is made all the more rapid. Meanwhile, technological advancements in the tools used to identify important molecular players and to tease out their exact roles is shifting the pace of discovery into entirely new gears.
And thus we find ourselves as we enter 2017, with many of the pieces in many important diseases discovered and those remaining to be placed before there are cures becoming smaller and smaller. Accordingly, our expectations for advancements in medicine over the next decade should be high. Very high. Already we see drugs targeting new molecular pathways, sometimes by making use of our growing understanding of our own body’s disease-fighting functions, entering the market. The Food and Drug Administration, cognizant of the deepening of our understandings and the importance of new discoveries has created a new regulatory pathway for breakthrough therapies. Some new products have made it from conception to market in a handful of years. This timeframe contrasts starkly with the decade historical medicines have required. Therefore not only will discoveries occur more quickly, but their approval for human use as well.
The stock market has not been sitting idly by. 2012 through 2015 were years in which the biotechnology indexes tripled. In the private markets, valuations of private biotechnology companies rose correspondingly. That is until 2016, when the biotechnology index fell approximately 20%, the potential for companies to go public changed abruptly, and funding for earlier stage enterprises began to become difficult again. What happened?
While the pace of invention is historic, that does not mean it will go in a straight line, or that everyone should have the stomach for the inevitable failures along the way. During the years of strong performance, many who lacked this perspective joined the joyful fray to participate in the gains. When the market retraced, however, they found themselves wondering if they could evaluate those companies in which they were invested, and the answer was often no. Thus, bad market performance led to worse performance by virtue of selling by those headed for the door. The party, after all, seemed over for now, particularly with a seemingly certain Clinton win on the way and all that could accompany that in regard to what was expected to be negative changes in drug pricing and reimbursement. The surprise Trump win caused a moment of reflection where prospects for biological innovators seemed brighter, followed by general confusion as campaign promises to overturn the Affordable Care Act became more muted and as bystanders waited to see what a Trump administration might actually look like.
This is all a side show in which it is easy to lose perspective. Throughout the world there are many different payment systems for new drugs. Most of them are government operated. Despite penny pinching and hurdles that have made drug launches more complex in certain countries, there are vanishing few instances where a drug that actually does something new and important receives an inadequate price. In the United States, whether you have a Hillary Clinton or a Donald Trump, this will be the case in the future. And the drugs that are to come will do important things.
What is more problematic are the disconnects between academic medicine and the commercial world. Academia is where most of the interesting new science is emanating. Yet in the academic setting, it is difficult to marshal all of the technical expertise and funding required to advance a medicine past the conceptual stages. Meanwhile, the venture industry and the pharmaceutical industry have sought to cherry pick the best academia has to offer. However, identifying the cherries is something no one has been good at, leaving behind a disappointing morass of missed opportunities, wasting assets and frustrated faculty.
We are proud this year to have announced the formation of Bridge Medicines, a bold attempt to overcome many of the barriers to translational research. This new company is a collaboration between ourselves, Takeda Pharmaceutical Company, Memorial Sloan Kettering Cancer Center, Weill Cornell Medicine and The Rockefeller University, along with Bay City Capital. Through this entity, the intellectual property from prominent academic institutions is moved forward through pharmaceutical technical expertise, and combined with funding and the ability to create new enterprises. In this manner, the barriers between a discovery and the ability to find the drug we need in our medicine cabinets, are removed.
We hope Bridge will not be just one piece of the puzzle, but rather a catalyst for bringing many pieces in the beautifully complex puzzle placed, oriented, and connected. Please keep reading to learn what makes Bridge so unique, and why it is an important new venture for advancing healthcare.